The enteric nervous system embedded in our gastrointestional tract is now recognized as a complex, integrative brain in its own right.
Ilya Mechnikov, a scientist arguably quite ahead of his time, shared a Nobel Prize in Physiology or Medicine in 1908 for his work on human immunity. His research interest was garnered by his (not arguable) horrid experiences with diseases caused by bacterial infection. After his first wife died from tuberculosis, he attempted to take his own life with an opium overdose, but managed to live. His misery didn’t end there however. When his second wife developed typhoid fever, he wanted to die with her, and inoculated himself with a tick-borne disease. They both survived, but it made him realize the salient significance of the body’s natural immune system.

In the wake of their bodies’ perseverance, Mechnikov grew dedicated—obsessed—with research in human immunity. During the cholera epidemic in France in 1892, as part of his self-experimentation, he drank a culture of Vibrio cholera, the bacteria responsible for the disease.
He didn’t get sick, so he gave the culture to a volunteer in his lab—who didn’t contract cholera either—but a second volunteer became stricken with the disease and subsequently died. In further lab experiments, he found that some microbes stimulated the growth of cholera bacteria and some hindered it.
He thought the human gut flora was responsible for this and hypothesized that if ingesting a pathogenic culture can make you sick, then surely a good one should promote health. “With the help of science man can correct the imperfections of his nature,” he wrote.
![]()
The use of fermented foods—which in essence and most cases are cultures of “good bacteria”—has been around for centuries. Research shows written records of the health benefits of fermented milk (yogurt) and fermented milk products date as far as back as 6000 BC in ancient Hindu scripts. The Greeks made written reference to fermented food products in 100 BC, and it is reputed that Genghis Khan fed his army fermented mare’s milk because he believed it instilled bravery in them. It was not until the 20th century, though, that a Bulgarian medical student—Stamen Grigorov—discovered a lactic acid bacteria (Bacillus bulgaricus) in yogurt cultures.

Bacterial fermented foods, thought to promote digestive health, are prevalent throughout history and ubiquitous in every corner of the globe, from Eastern European sauerkraut to Korean kimchi to Japanese natto.
In current times, a growing body of research shows that maintaining a healthy gut and microbiome with diet can have a significant impact on health and well being. Further, it is contended that the right balance of gut bacteria can help stave off disease.
The Second Brain In Our Gut
A microbiologist once told me, “You are over 90% bacteria and about 10% human.” What? Really? I then looked it up and found out it was indeed true. We have 10 times more bacterial cells cohabiting our bodies than human cells. The human microbiome is collectively the 10-100 trillion microorganisms, mainly bacteria, living in our gut weighing between one to three pounds; every microbiome is specific to a particular environment (all microorganisms interacting with each other in a particular area in the body, such as the gastrointestinal tract or gut) or body part.
The microbiome also refers to the combined genetic material of the microbiota in that environment or organ. These trillions of bacteria interact and communicate with the enteric nervous system or what scientists have labeled the “second brain.”
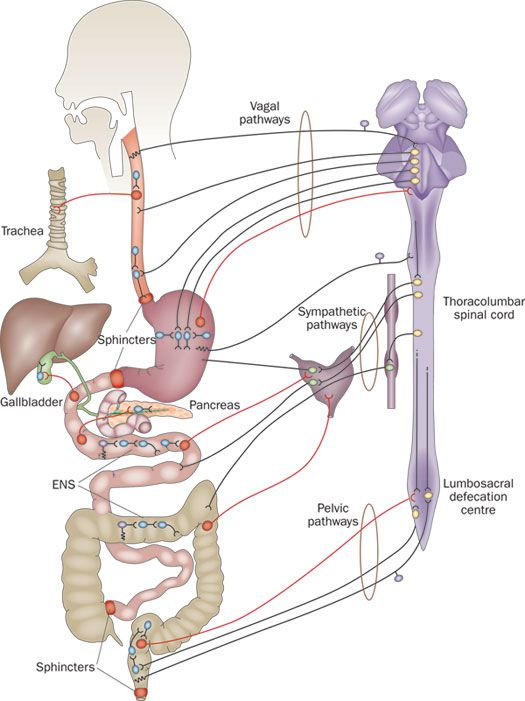
As Michael Gershon, Professor of Pathology and Cell Biology at Columbia University and “father of neurogastroenterology” writes, “Once dismissed as a simple collection of relay ganglia (a cluster of nerve cell bodies), the enteric nervous system is now recognized as a complex, integrative brain in its own right.”
The enteric nervous system comprises about 500 million neurons or two-thirds the amount found in a cat. It is embedded in our gastrointestinal tract—starting at the esophagus and ending at the anus.
Together, the second brain in the gut and its microbiome have a significant effect on the brain, influencing mood, behavior, and disease. As such, the National Institute of Health (NIH) in the U.S. launched the Human Microbiome Project in order to identify and characterize the human microbiota.
The Great Brain-Gut-Microbiome Connection
Recent evidence shows that the brain interacts with the enteric system in the gut (second brain) and the gut microbiome in a bi-directional manner. It’s a three-way communication circuit called the Brain-Gut-Microbiome Axis and involves three systems—central nervous, gastrointestinal, and immune. A common example of a brain-gut interaction is that “butterflies in your stomach” feeling. Your palms are sweaty and trembling, your heart rate increases, your skin becomes pale or flushed, and you (maybe) feel like you want to throw up. This is part of the fight-or-flight physiological stress response and evidence of how our gut is related to how we feel.
So just how do the microbes in our gut influence mood? In truth, scientists are not 100% clear on how these microbes directly influence our brains, but they propose that it’s through multiple pathways. For example, it is well known that the four main chemicals associated with happiness and mood in human beings are dopamine, serotonin, oxytocin, and endorphins. Research shows serotonin (although its function is complex as it is involved in many physiological processes) to be a mood regulator playing a major role in the treatment of depression and susceptibility to both depression and suicide.
The enteric nervous system in our gastrointestinal tract is now recognized as a complex, integrative brain in its own right. Click To TweetAnd guess what? About 90% of the body’s serotonin is made in the digestive tract; researchers at Caltech showed that gut microbes are integral in serotonin synthesis. Similarly, gut bacteria are implicated in the synthesis of other chemicals and neurotransmitters involved in mood regulation and disease. Further, scientific findings in the journal Behavioural Brain Works illustrated that some bacteria affect how these mood compounds are metabolized. Perhaps the most fascinating finding, however, is that some microbes can activate the vagus nerve, the longest cranial nerve in the body and a main line of bidirectional communication between the brain and gut.
90% of the body’s serotonin is made in the digestive tract. Click To TweetAdvancements in genome sequencing technology is enabling research into the impact of the gut microbiome on disease. By mapping the genome of the gut microbiome in diseased vs. healthy humans and animals, conclusions on the role of the gut microbes in disease proliferation can—and have been—deduced. And a very interesting way to glean this information is to analyze feces.
Your poop can be very informative. One study using genetic analysis of clinically depressive folks’ poop compared to those who aren’t, found several correlations between the human fecal microbiota (representative of gut microbiota) and depression. Although they report that their findings need to be further tested in larger cohorts, their results were specific for a particular strain (sub-type of microorganism) and genus (group or class of species):
“The Oscillibacter type strain has valeric acid as its main metabolic end product, a homolog of neurotransmitter GABA (γ-aminobutyric acid), while Alistipes has previously been shown to associated with induced stress in mice.”
“Valeric acid structurally resembles GABA, and has been shown to bind the GABAa receptor. Therefore, it is possible that bacteria involved in valeric acid production and/or metabolism could also be associated with depression.”
The work of professor Bernhard Lüscher and colleagues at Penn State University shows that enhancing the activity of GABA in the brains of depressed mice has antidepressive effects, similar to that of antidepressive drugs, bringing mice back to “normal” behavior. Noteworthy is that GABA is implicated in mood disorders and its agonists have been shown to be antidepressive and antimanic.
This includes less of a certain type of bacteria in human fecal microbiota in depressed individuals compared to healthy ones.
Your poop can be *very* informative. Studies have found several correlations between the human fecal microbiota and depression. Click To Tweet
What is even more interesting is that researchers at the University College Cork in Ireland showed that if you transplant the microbiome from a depressed individual to animals, these animals will exhibit the same behavior of the depressed individual. These include anhedonia (not wanting to do the things you usually take pleasure in) and anxiety-like behavior.
This study also showed that depression is associated with decreased gut microbiota richness and diversity. Further, in humans, fecal microbiota transplants—introducing healthy feces into the microbiome of a diseased person—have been successful in the treatment of gastrointestinal disease and colitis and clostridium difficile infection with an efficacy rate of almost 90%.
Auxiliary Advances
Across the globe, the International Diabetes Federation report 425 million adults (or 1 in 11 adults) with diabetes while the CDC reports 30.3 million in the U.S. (with over 100 million living with diabetes or prediabetes) and 3.4 million in Canada, according to Diabetes Canada.
Diabetes and obesity are oftentimes linked as it is well-documented that obesity has a strong correlation between insulin resistance and diabetes. In a promising and growing area of research using humans, a small clinical trial in the Netherlands showed that a fecal transplant from a lean donor can temporarily improve insulin resistance in obese men.
![]()
In another growing area of research, large differences are seen in the gut microbiomes of people with Parkinson’s disease compared with healthy individuals. Further, a study published in the journal Cell show that when fecal microbes from persons with Parkinson’s disease was transferred to mice, they exhibited more severe symptoms of the disease and the aggregation of α-synuclein in the brain. (The formation of plaques in the brain via aggregation of α-synuclein is found in persons with neurodegenerative diseases including Parkinson’s, Alzheimer’s and dementia.) Meanwhile another study revealed that probiotic supplementation in patients with Alzheimer’s disease showed improved in cognitive function.
But Just How Did We Get Our Microbiome Anyway?
It has been shown that the vaginal and maternal gut microbiome changes significantly during pregnancy. Science journal PLOS|One as well as a Finnish study in Cell, respectively, showed that pregnant women exhibit lower vaginal bacteria than nonpregnant women as well as a lack of population diversity in gut microbiota.
In the International Journal of Obesity, researchers found that children exposed to prenatal antibiotics in the second or third trimester had an 84% higher risk of developing obesity compared to children who were not exposed. Further, Caesarians were linked to 46% higher risks of developing childhood obesity; your first microbe colonizers are acquired via exchanges with your mother largely during the birthing process, when you are—quite literally—slathered in vaginal bacteria.
In addition, as research shows, any disturbance to this microbe exchange such as delivery by C-section, perinatal antibiotics and formula feeding is linked to an increased risk in metabolic and immune disease. After birth, a child’s microbiome continues to grow and is changed by ingestion of the microbes in breast milk which stabilizes neonatal gut microbiome. As we grow older, our gut microbiomes can change throughout life depending on diet, environment and the drugs we may take, such as antibiotics.
So How Do We Promote A Healthy Microbiome?
There is still a lot of ongoing research on the brain-gut-microbiome connection, but it’s quite clear that the gut microbiota can have a significant impact on mood, health and disease. So here are a few ways—and foods!—that will keep your gut microbiome healthy and thriving:
- Probiotics – research shows they can be used to maintain a healthy gut and restore the gut microbiota to health (after disruption as in the case of illness and the use of antibiotics).
- Prebiotics foods – these cause the growth and stimulate the activity of beneficial microbes in the gut.
- Whole grain foods/foods high in fiber – these have been shown to promote the growth of specific bacteria only digested by certain bacterial types. For example, apples and artichokes have been shown to increase Bifidobacteria (a good bacteria) in humans.
- Fermented foods – people have been eating these foods for centuries. They have been shown to reduce the number of disease-causing bacteria in the gut and promote the growth of beneficial bacteria. It has been shown that people who eat a lot of yogurt have less of the bacteria linked to inflammation (Enterobacteriaceae).
- Diversity in food – a diet comprising a diversity in food leads to a diverse microbiota which is considered healthy.
- Polyphenols in red wine and grapes – these have been shown to improve specific beneficial microbiota.

